Eastern Narrow-mouthed Toad
Gastrophryne carolinensis
Common Name: |
Eastern Narrow-mouthed Toad |
Scientific Name: |
Gastrophryne carolinensis |
Etymology: |
|
Genus: |
Gastrophryne is derived from the Greek words gaster meaning "belly" and phrynos meaning "toad". |
Species: |
carolinensis refers to South Carolina, where the toad was first found and described. |
Average Length: |
0.9 - 1.3 in. (2.2 - 3.2 cm) |
Virginia Record Length: |
|
Record length: |
1.5 in. (3.8 cm) |
Physical Description - This is a small species with a length of 22-38 mm (7/8 to 1.5 in). It has smooth skin, a pointed snout, and a distinctive fold of skin across the bakck of the head. The dorsoum varies from gray to brown to reddish in color. The typical pattern is a broad stripe down the center with light stripes down the sides. The pattern is often broken up by mottling or spotting. The coloring may change with the individual's environment. The venter is heavily speckled or mottled, and the males usually have dark throats. This toad species is also distinctive for its lack of parotoid glands, warts, and webbed toes.
Historical versus Current Distribution - Eastern Narrow-mouthed Toads (Gastrophryne carolinensis) occur in the southeastern and lower midwestern United States, from Maryland south to the Florida Keys, west to central Texas, and north to Kentucky, southern Illinois, southern Missouri, and extreme southeastern Nebraska (Carr, 1940a; Wright and Wright, 1949; Duellman and Schwartz, 1958; Nelson, 1972c; Harris, 1975; Martof et al., 1980; Ashton and Ashton, 1988; Dundee and Rossman, 1989; Conant and Collins, 1991; Redmond and Scott, 1996; Bartlett and Bartlett, 1999a; Mitchell and Reay, 1999; Phillips et al., 1999; Dixon, 2000; Johnson, 2000). Minton (2001) speculated that this anuran may occur in Indiana. They reach elevations to 549 m in the Smoky Mountains National Park (Huheey and Stupka, 1967), although they are absent from most of the Blue Ridge Mountains and the Appalachian region north of Tennessee (Redmond and Scott, 1996). Nelson (1972c) noted that they occur as high as 732 m in Oklahoma. Eastern Narrow-mouthed Toads also occur on numerous barrier islands in the Gulf Coast and off the southeastern Atlantic Coast (Engles, 1952; Blaney, 1971; Gibbons and Coker, 1978; Braswell, 1988; Learm et al., 1999). The disjunct population in southeastern Iowa may be extirpated (Klimstra, 1950; Nelson, 1972c).
Historical versus Current Abundance - Eastern Narrow-mouthed Toads are solitary, secretive, and common throughout Alabama (Mount, 1975), Florida (Carr, 1940a), and Louisiana (Penn, 1943; Anderson, 1954), and locally common in Illinois (Phillips et al., 2000) and Virginia (Hoffman and Mitchell, 1996). Eastern Narrow-mouthed Toads are commonly captured in drift fences with pitfall traps (Gibbons and Bennett, 1974; Buhlmann et al., 1994; Enge, 1997). Some studies have revealed large numbers in local populations (e.g., Bennett et al., 1980; Gibbons and Semlitsch, 1981; Dodd, 1992, 1995b), although annual variation in numbers encountered varies among sites and years (Dodd, 1995b; Semlitsch et al., 1996; Enge, 1997).
Abundance estimates or counts are highly dependent on the sampling technique used. Funnel traps along drift fences revealed few individuals in a study in northern Florida (Vickers et al., 1985). Lamb et al. (1998) reported only 19 eastern narrow-mouthed toads among 1,070 frogs encountered under coverboards in a North Carolina floodplain. Coverboards in South Carolina produced few captures, whereas drift fences with pitfall traps yielded thousands of captures over the same time period (Grant et al., 1992). However, Grant et al. (1994) recorded high numbers of narrow-mouthed toads under coverboards in different aged pine stands. Other studies reporting high numbers of captures using drift fences with pitfall traps include Bennett et al. (1980), Gibbons and Semlitsch (1981), Dodd (1992), Enge (1997), and Hanlin et al. (2000). More individuals of this species were trapped in a deep ditch in Louisiana than any other amphibian (Anderson, 1952).
Changes in local abundance have been little documented. Fowler and Stine (1953) noted the reduction of suitable habitat for this species in southern Maryland due to ditching, agriculture, and logging. Forestry operations may affect abundance in some areas, as more individuals were captured in a naturally regenerated slash pine forest than in two sites intensively prepared for pine plantations in Florida (Enge and Marion, 1986). There was no successful reproduction in a small pond created for mitigation in South Carolina despite the abundance of adults that used this habitat for several years following construction (Pechmann et al., 2001). Delis et al. (1996) found that narrow-mouthed toads were less abundant after urbanization occurred at a Tampa, Florida, area, but were the most abundant species in a nearby, relatively undisturbed, pine flatwoods habitat.
Breeding - Reproduction is aquatic.
Breeding migrations - Breeding occurs from March–October in southern populations but is more restricted in the northern portion of its range (e.g., Barbour, 1941; Wright and Wright, 1949; Brandt, 1953; Hoffman, 1955; Martof, 1955; Duellman and Schwartz, 1958; Nelson, 1972c). Calling is usually initiated by heavy rains. Eastern Narrow-mouthed Toads call from April–September in Alabama (Mount, 1975); 1 April–3 September in Florida (Carr, 1940a; Einem and Ober, 1956); May–August in Georgia (Wright, 1932); late March to early October in Louisiana (Anderson, 1954; Dundee and Rossman, 1989); 30 March–September in eastern North Carolina (Funderburg, 1955); late spring to September in South Carolina (Gibbons and Semlitsch, 1991; Hall, 1994); and May–August in Virginia (J.C.M., personal observations). Terrestrial activity in Virginia extends from 28 April–6 October (Hoffman and Mitchell, 1996). Mittleman (1950) notes that breeding males exhibit the secondary sexual characters of enlarged tubercles on the chin and anterior edge of the lower jaw.
Breeding habitat - Males call from temporary ponds, flooded pastures, shallow depressions in open fields, rain-filled ditches, edges of permanent ponds, and open grassy habitats, usually while partially submerged or hidden under grasses and other vegetation or sitting in the mouths of their burrows (Allen, 1932; Brandt, 1936a; Gosner and Black, 1956; Gibbons and Semlitsch, 1991). Males conceal themselves in grass and under vegetation and debris while calling (Carr, 1940a). They often call with just their noses protruding above the water line and are very difficult to observe. Wright (1932) observed males wiggle their bodies into the wet sand and call with only their snouts protruding.
Male mating calls and calling behavior have been described by Wright (1932), Wright and Wright (1949), Anderson (1954), Nelson (1973), and Conant and Collins (1991), among others. The breeding call sounds like the strong bleat of a lamb. Males apparently do not grip females during amplexus, although Anderson (1954) noted strong axillary amplexus in laboratory matings. Special glands in the sternal region of males secrete a substance that allows adhesion of breeding pairs (Conaway and Metter, 1967; Holloway and Dapson, 1971).
Egg deposition sites - Females deposit a small sheet of eggs on the water's surface in thick, grassy vegetation or in smaller packets of 10–90 eggs in highly ephemeral pools of water (Wright, 1932; Wright and Wright, 1949; Gibbons and Semlitsch, 1991). Wright (1932) found some attached to vegetation 2.5–5 cm (1–2 in) under water.
Clutch size - Wright and Wright (1949) reported a total of 850 eggs in southeastern Georgia, and Mitchell (1986) noted a total of 1,600 eggs in Virginia. Counts of ovarian follicles ranged from 152–1,089 for a sample of 66 females from Louisiana (Anderson, 1954) and 186–1,459 for 24 females in Arkansas (Trauth et al., 1999).
Altig & McDiarmid 2015 - Classification and Description:
- Eastern Film
- Arrangement 1 - Eggs deposited in ephemeral nonflowing water; eggs in coherent films, individual eggs easily pushed apart; upper hemisphere of jelly of recently deposited eggs projects above water surface, film diameter less than 150 mm; ovum intensely black and jelly diameter large relative to ovum size.
Length of larval stage - Eggs hatch in 1–1.5 d (Wright, 1932; Orton, 1946). Tadpoles metamorphose 20–70 d after egg deposition (Wright, 1932; Martof et al., 1980). Larval development occurs quickly, from having "hindlimbs well developed" to complete metamorphosis in 6–10 d (Anderson, 1951). Wright (1932) pointed out the ephemeral nature of the breeding pools and the high rates of mortality due to rapid pool drying.
Tadpoles:
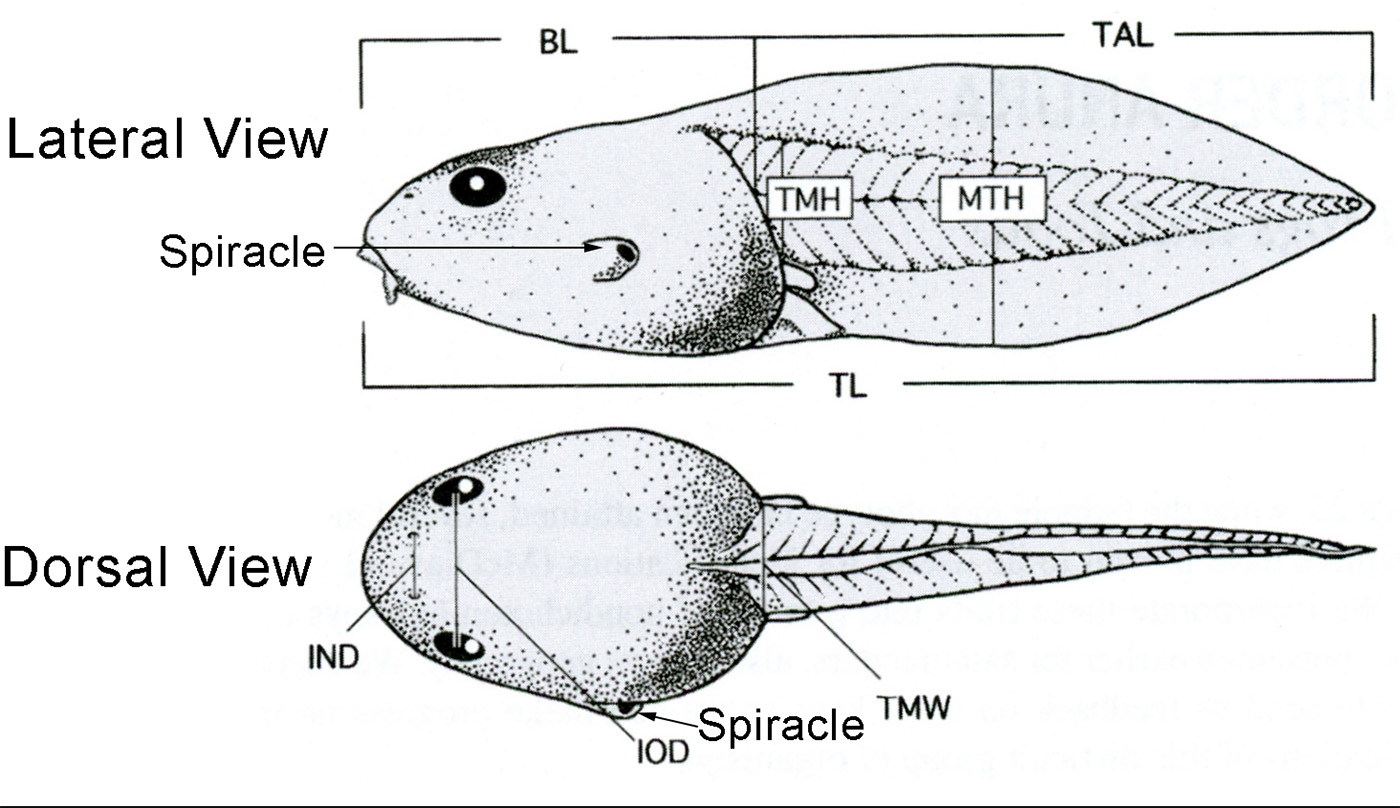
| Lateral View | Dorsal View |
|---|---|
| BL = Body Length | IND = Internarial Distance |
| MTH = Maximum Tail Height | IOD = Interorbital Distance |
| TAL = Tail Length | TMW = Tail Muscle Width |
| TL = Total Length | |
| TMH = Tail Muscle Height |
Larvae Food - Eastern Narrow-mouthed Toad tadpoles are planktonic feeders but lack the keratinized mouthparts characteristic of other tadpoles in eastern North America (Altig and Kelly, 1974).
Cover - Tadpoles will hide under bottom debris and leaves if they are present, but these larvae often occur in pools without any cover on mud to hard clay bottoms (Anderson, 1954; J.C.M., personal observations).
Larval polymorphisms - Unknown and unlikely
Features of metamorphosis - Size at metamorphosis was 11–13 mm SVL in southeastern Georgia (Wright, 1932), 10–12.5 mm in Louisiana (Orton, 1946; Anderson, 1954), 11 mm in northern Florida (Dodd, 1995b), and 8 mm in southeastern Virginia (Mitchell et al., 1998). Timing of metamorphosis was mid June to September in Georgia (Wright, 1932), May–October in Louisiana (Anderson, 1954), and 26 August–9 September in Virginia (Mitchell, 1986).
Post-metamorphic migrations - Dodd and Cade (1998) determined that movement patterns of eastern narrow-mouthed toads into and out of a breeding pond in Florida were nonrandom in orientation. Juvenile frogs emigrated toward high pine habitats rather than to xeric oak hammocks and grass meadows.
Juvenile Habitat - Juveniles have been found in the same habitats as adults, although Dodd and Cade (1998) found differences in habitats selected by emergent juveniles and older juveniles and adults immigrating to the breeding pond in northern Florida.
Adult Habitat - Eastern Narrow-mouthed Toads are secretive and highly terrestrial. The related aspects of cover and moisture are the two most important environmental variables influencing their presence (Carter, 1934; Robeson and Tyson, 1950; Anderson, 1954; Martof, 1955). General habitat types include cypress-gum swamps, bottomland hardwoods, live-oak ridges, pine-oak uplands, sandy woodlands and hillsides, open woods, prairies, mixed hardwoods, pine forests, longleaf pine sandhills, riparian floodplains, brackish marshes, coastal secondary dune scrub forest, and maritime forests (Blanchard, 1922; Wright, 1932; Brandt, 1936a; Wood, 1948; Blair, 1950; Werler and McCallion, 1951; Anderson, 1954; Tinkle, 1959; Dodd, 1992; Buhlmann et al., 1994; Learm et al., 1999).
Eastern Narrow-mouthed Toads appear to remain in the vicinity of breeding pools awaiting high humidity and heavy rains (Brandt, 1936a). Cover objects such as rocks, decaying logs, mats of vegetation, bark of logs and stumps, and boards along the edges of ponds and streams are often used for shelter (Holbrook, 1842; Wright, 1932; J.C.M., personal observations). Eastern narrow-mouthed toads may also use crayfish burrows, loose leaf mold, and other vegetation for shelter (Carr, 1940a). Goin (1943) found them under mats of dead water hyacinths that had been tossed on the shore. They have been found around human dwellings, often being taken under trash and boards (Duellman and Schwartz, 1958). Duellman and Schwartz (1958) noted a single individual taken from a wood rat (Neotoma floridana) nest on Key Largo.
Adults are tolerant of brackish water (Noble and Hassler, 1936; Hardy, 1953; Conant, 1958b; Neill, 1958a). Their ability to tolerate and disperse over saltwater has allowed them to colonize barrier islands and live in brackish marsh habitats.
Home Range Size - Tinkle (1959) found that eastern narrow-mouthed toads marked over a 5 mo period in Louisiana moved no more than 3 m and stayed within the same 5-m quadrant. Dodd (1996) reported movement distances away from a breeding pond in Florida of 42–914 m. Males move more than females (Anderson, 1954).
Territories - Eastern Narrow-mouthed Toads are not known to establish territories. Anderson (1954) noted that calling positions are as close as 2 cm or as distant as several meters apart.
Aestivation/Avoiding Dessication - Werler and McCallion (1951) noted a "spittle-like" substance on a narrow-mouthed toad found in summer under a piece of cardboard in sand and suggested that it may have been secreted by the individual frog. However, true aestivation has not been documented. Individuals occasionally seek refuge behind and in between dead fronds of cabbage palms during droughts (Lee, 1969a).
Seasonal Migrations - Eastern Narrow-mouthed Toads are active during most warm months and may be found in all months of the year in the southern portions of its range. Dodd (1995b) found them in all months, although adult activity was reduced October–April. Migrations to breeding sites are less influenced by seasonal conditions than by rainfall.
Torpor (Hibernation) - Eastern Narrow-mouthed Toads overwinter in rotten logs, beneath bark of pine stumps as high as 1 m above ground, buried in sand at the base of small, fallen trees, and under leaf litter (Neill, 1948b; Engles, 1952). Individuals are found occasionally behind and between overlapping dead fronds at the base of cabbage palms during cold periods in northern Florida (Lee, 1969a).
Interspecific Associations/Exclusions - Eastern Narrow-mouthed Toads breed in association with northern cricket frogs (Acris crepitans), green treefrogs (Dryophytes cinerea), Cope's gray treefrogs (Dryophytes chrysoscelis), pine woods treefrogs (Dryophytes femoralis), squirrel treefrogs (H. squirella), gray treefrogs (H. versicolor), Fowler's toads (B. fowleri), oak toads (Bufo quercicus), southern toads (Bufo terrestris), Gulf Coast toads (B. nebulifer), and eastern spadefoot toads (Scaphiopus holbrookii), as well as eastern newts (Notophthalmus viridescens), marbled salamanders (Ambystoma opacum), mole salamanders (Ambystoma talpoideum), tiger salamanders (Ambystoma tigrinum), and dwarf salamanders (Eurycea quadradigitata; Barbour, 1941; Wright and Wright, 1949; Gordon, 1955; Hoffman, 1955; Volpe, 1956; Mount, 1975; Bennett et al., 1980; Pechmann et al., 2000; J.C.M., personal observation). Pechmann et al. (2001) found the following species of anurans in the same ponds with eastern narrow-mouthed toads in South Carolina: northern cricket frogs, oak toads, southern toads, Cope's gray treefrogs, pine woods treefrogs, barking treefrogs (Dryophytes gratiosa), spring peepers (Pseudacris crucifer), southern chorus frogs (Pseudacris nigrita), ornate chorus frogs (Pseudacris ornata), American bullfrogs (Rana catesbeiana), green frogs (Rana clamitans), southern leopard frogs (Rana sphenocephala), and eastern spadefoot toads (Scaphiopus holbrookii). Carr (1940a) found adults with introduced geckos and native skinks under debris in Key West.
Eastern Narrow-mouth Toads occasionally hybridize with Great Plains narrow-mouthed toads (G. olivacea) along their zone of sympatry in eastern Oklahoma and Texas (Hecht and Matalas, 1946; Nelson, 1972c). Studies of call patterns, larval development, and body size variation in the overlap zone by A.P. Blair (1950, 1952) and W.F. Blair (1955a,b) indicated that differences are more pronounced in sympatry than in allopatry and that interspecific hybridization, when it occurs, is reinforced by isolating mechanisms (Nelson, 1972c). Volpe (1956) reported finding two mating pairs of male squirrel treefrogs with female eastern narrow-mouth toads and three pairs of male eastern narrow-mouth toads with female squirrel treefrogs in New Orleans, although none of the eggs were fertile.
Age/Size at Reproductive Maturity - Adult body sizes range from 20–36 mm SVL (Wright, 1932; Wright and Wright, 1949). Females usually attain larger sizes than males. Wright (1932) noted that males matured in the Okefenokee Swamp in Georgia at 21 mm and females at 22 mm. Maximum body size was 29 mm for males, 30 mm for females. Anderson (1954) noted that males in Louisiana had mature spermatozoa at 18.4 mm, females had pigmented eggs at 23 mm. In southern Florida, males measured 18.8–30.5 mm and females were 22.4–32.5 mm (Duellman and Schwartz, 1958). In northern Florida, males measured 22–34 mm and females 21–35 mm (Dodd, 1995b). Maximum body sizes for narrow-mouthed toads in Louisiana was 32.2 mm for males and 34.3 for females (Anderson, 1954), and 29 mm for males and 32 mm for females in a central Virginia population (Mitchell, 1986). Trauth et al. (1999) reported size at maturity for males in Arkansas at 21.5 mm and females at 26.7 mm, both in their second year of life. He also noted maximum body sizes of 33.6 mm and 36.5 mm, respectively. In Louisiana, males first reproduce after a year of post-metamorphic growth; females may reach maturity at either 1–2 yr (Anderson, 1954).
Longevity - Conant and Hudson (1949) reported 6 yr, 9.5 mo for a wild-caught adult. Dodd (1995b) documented a wild-caught individual in its fourth year in northern Florida and noted that average life spans under natural conditions remain unknown.
Feeding Behavior - Although this small frog will consume a wide variety of prey, ants, termites, and small beetles are the primary taxa in most stomachs (Wood, 1948; Anderson, 1954; Martof, 1955; Duellman and Schwartz, 1958). Other prey include small (maximum length 6.3 mm) snails, isopods, spiders, mites, collembolans, and lepidopterans, many of which are secretive (Anderson, 1954).
Predators - Known predators of adults are Glossy Watersnakes (Regina rigida), Eastern Garter Snakes (Thamnophis sirtalis), Copperheads (Agkistrodon contortrix), Cottonmouths (Agkistrodon piscivorus), and cattle egrets (Bubulcus ibis; Wright, 1932; Anderson, 1942; Hamilton and Pollack, 1955, 1956; Jenni, 1969; Trauth and McAllister, 1995).
Anti-Predator Mechanisms - Eastern Narrow-mouthed Toads avoid predators by burrowing and seeking cover, and by nocturnal activity patterns. Carr (1940a) described them as "nimble and active when frightened." He noted that they dive into the mouths of crayfish burrows after exposure of their hiding places under logs, and that they rapidly burrow out of sight in loose leaf mold. Mucous secretions produce a violent burning sensation to one's eyes, irritate membranes in the mouth and throat, and may be toxic to other amphibians (Anderson, 1954). He described how one frog's secretions caused attacking ants (Iridomyrmex sp.) to become entangled in the thick layer on the skin and that the ants were brushed off as the frog dove under a lump of dirt. Garton and Mushinsky (1979) demonstrated that the skin secretions were unpalatable and deterred predators.
Diseases - Unknown.
Parasites - Unknown.
Conservation - The northernmost populations of Eastern Narrow-mouthed Toads known are in Maryland. Their listing as a state Endangered species here reflects loss of habitat due to urban sprawl and other ways the landscape is affected by human activities (Fowler and Stine, 1953; Harris, 1975; Levell, 1997).
References for Life History
- Altig, Ronald & McDiarmid, Roy W. 2015. Handbook of Larval Amphibians of the United States and Canada. Cornell University Press, Ithaca, NY. 341 pages.
- AmphibiaWeb. 2020. University of California, Berkeley, CA, USA.
- Conant, Roger and, Collins, John T., 2016, Peterson Field Guide: Reptiles and Amphibians, Eastern and Central North America, 494 pgs., Houghton Mifflin Company., New York
- Duellman, William E. and, Trueb, Linda, 1986, Biology of Amphibians, 671 pgs., The Johns Hopkins University Press, Baltimore
- Martof, B.S., Palmer, W.M., Bailey, J.R., Harrison, III J.R., 1980, Amphibians and Reptiles of the Carolinas and Virginia, 264 pgs., UNC Press, Chapel Hill, NC
- Wilson, L.A., 1995, Land manager's guide to the amphibians and reptiles of the South, 360 pp. pgs., The Nature Conservancy, Southeastern Region, Chapel Hill, NC
Photos:
*Click on a thumbnail for a larger version.
Verified County/City Occurrence
Accomack
Amelia
Appomattox
Brunswick
Buckingham
Campbell
Caroline
Charles City
Charlotte
Chesterfield
Cumberland
Dinwiddie
Essex
Franklin
Gloucester
Goochland
Greensville
Halifax
Hanover
Henrico
Henry
Isle of Wight
James City
King and Queen
King William
Lancaster
Lee
Lunenburg
Mathews
Mecklenburg
Nelson
New Kent
Northampton
Pittsylvania
Powhatan
Prince Edward
Prince George
Scott
Southampton
Surry
Sussex
Westmoreland
York
CITIES
Chesapeake
Colonial Heights
Danville
Hampton
Newport News
Petersburg
Poquoson
Portsmouth
Suffolk
Virginia Beach
Verified in 43 counties and 10 cities.
U.S. Range









